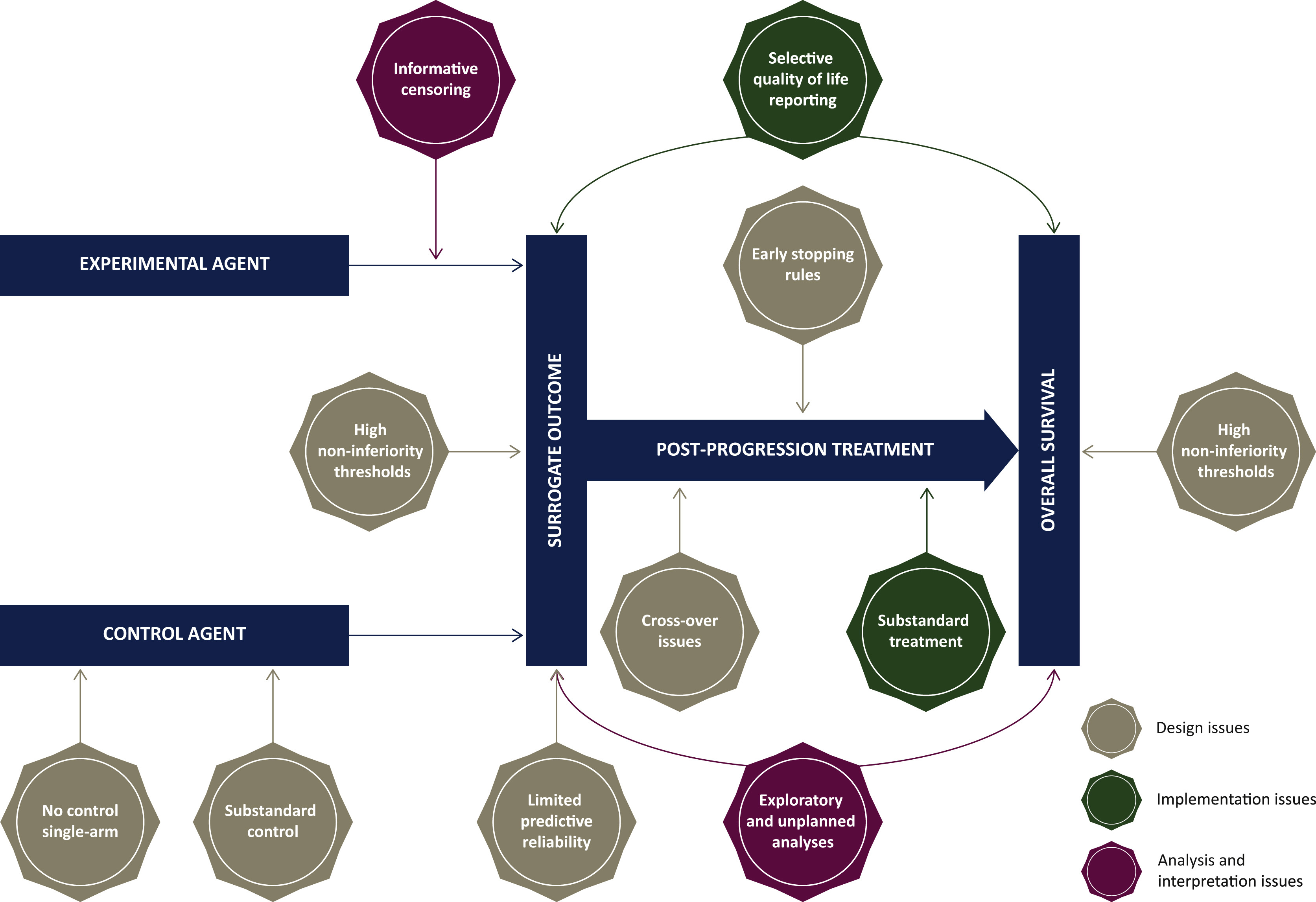
Figure from Gyawali et al., 2021, highlighting issues in study design, implementation, and data analysis that may alter the results of a trial.
Our team has extensively published on the critical appraisal of cancer drug clinical trials – both general lessons and trial-specific examples. Clinical practice dictated by poorly designed, conducted, or reported clinical trials is not in the best service of patients. Thus, critical appraisal and feedback on how to properly design, conduct, and report clinical trials is a core component of our work. Besides publications, Dr. Gyawali also serves this mission through his involvement in the ESMO Magnitude of Clinical Benefit Scale working group. Our work has highlighted several shortcomings of cancer clinical trials such as inadequate measurement and reporting of quality of life data, substandard comparator arms, financial conflicts of interest, industry involvement, a lack of population diversity in clinical trials, informative censoring, pitfalls of subgroup analysis and non-inferiority design trials, a lack of transparency in reporting harms, and the use of non-validated surrogate endpoints.
Relevant Publications:
- Tannock IF, Goldstein DA, Ofer J, Gyawali B, Meirson T. Evaluating Trials of Adjuvant Therapy: Is There Benefit for People With Resected Renal Cancer? J Clin Oncol. 2023 PMID: 36961983.
- Oosting SF, Barriuso J, Bottomley A, Galotti M, Gyawali B,…Piccart M, Cherny NI. Methodological and reporting standards for quality of life data eligible for European Society for Medical Oncology-Magnitude of Clinical Benefit Scale (ESMO-MCBS) credit. Annals of Oncology. 2022 December 19. PMID: 36549587
- Sachdev A#, Sharpe I, Bowman M, Booth CM, Gyawali B*. Objective response rate of placebo in randomized controlled trials of anticancer medicines. EClinicalMedicine. 2022 Nov 24;55:101753. PMID: 36444211. Altmetric score: 97.
- Poudyal B, Dulal S, Shilpakar R and Gyawali B* (2022) Highlights from ecancer Choosing Wisely Nepal 2022: critical appraisal skills for evidence-based practice, 24th–25th September 2022, Kathmandu, Nepal ecancer 16 1478. PMID: 36819797
- Akhade A, Van Wambeke S and Gyawali B* CDK 4/6 inhibitors for adjuvant therapy in early breast cancer—Do we have a clear winner?ecancer 2022. PMID: 36200010
- Covered by Medscape.
- Gyawali B*, Booth CM. Defining clinically important overall survival thresholds: lessons from quality of life. Nat Rev Clin Oncol. 2022 Jul 27. PMID: 35896739. Altmetric score: 59
- Strzebonska K, Blukacz M, Wasylewski MT, Polak M, Gyawali B, Waligora M. Risk and benefit for umbrella trials in oncology: a systematic review and meta-analysis. BMC Med. 2022 Jul 8;20(1):219. PMID: 35799149
- Lythgoe MP, Desai A, Gyawali B, Savage P, Krell J, Warner JL, Khaki AR. Cancer Therapy Approval Timings, Review Speed, and Publication of Pivotal Registration Trials in the US and Europe, 2010-2019. JAMA Netw Open. 2022 Jun. PMID: 35687337.
- Samuel JN#, Booth CM, Eisenhauer E, Brundage M, Berry SR, Gyawali B*. Association of Quality-of-Life Outcomes in Cancer Drug Trials With Survival Outcomes and Drug Class . JAMA Oncol. 2022 Apr. PMID: 35482347. Altmetric score: 182
- Kartolo A# and Gyawali B*. Should the control arms of randomized trials have an expiry date? Nat Rev Clin Oncol. 2022 Apr 1. PMID: 35365795.
- Li X, Beeghly-Fadiel A, Bhavnani SK, Tavana H, Rubinstein SM, Gyawali B, Riaz IB, Fernandes HD, Warner JL. Evaluation of Information Theoretic Network Meta-analysis to Rank First-Line Anticancer Regimens for Hormone Receptor-Positive, ERBB2-Negative Metastatic Breast Cancer. JAMA Network Open. 2022 Apr 1;5(4):e224361. PMID: 35416993
- Wells JC, Fundytus A, Sharma S, Hopman WM, Del Paggio JC, Gyawali B, Mukherji D, Hammad N, Pramesh CS, Aggarwal A, Sullivan R, Booth CM. Randomized Controlled Trials in Lung, Gastrointestinal, and Breast Cancers: An Overview of Global Research Activity. Curr Oncol. 2022 Apr 7;29(4):2530-2538. PMID: 35448181
- Gyawali B, Eisenhauer E, Tregear M, Booth CM. Progression-free survival: it is time for a new name. Lancet Oncol. 2022 Mar. PMID: 35240080. Altmetric Score: 128
- Wesson W, Galate VL, Sborov DW, McClune B, Goodman AM, Gyawali B, Prasad V, Abbasi S, Mohyuddin GR. Characteristics of clinical trials for haematological malignancies from 2015 to 2020: A systematic review. Eur J Cancer. 2022 Feb. PMID: 35236569.
- Van Wambeke S, Vera-Badillo FE, Gyawali B. Controlling the Control Arm in Metastatic Castration-Resistant Prostate Cancer Trials: Best Standard of Care or the Minimum Standard of Care? J Clin Oncol. 2022 Feb 21. PMID: 35188830. Altmetric Score: 71
- Dodkins J, Hopman WM, Wells JC, Lievens Y, Malik RA, Pramesh CS, Gyawali B, Hammad N, Mukherji D, Sullivan PR, Parkes J, Booth CM, Aggarwal A. Is Clinical Research Serving the Needs of the Global Cancer Burden? An analysis of contemporary global radiotherapy randomised controlled trials. Int J Radiat Oncol Biol Phys. 2022 Feb 10. PMID: 35151802.
- Gyawali B, Carson L, Berry S and Moraes F. Challenges of globalization of cancer drug trials- recruitment in LMICs, approval in HICs. The Lancet Regional Health – Americas. 2022. Altmetric score: 51
- Fundytus A, Wells JC, Sharma S, Hopman WM, Del Paggio JC, Gyawali B, Mukherji D, Hammad N, Pramesh CS, Aggarwal A, Sullivan R, Booth CM. Industry Funding of Oncology Randomised Controlled Trials: Implications for Design, Results and Interpretation. Clin Oncol (R Coll Radiol). 2021. PMID: 34479769.
- Van Wambeke S and Gyawali B. Atezolizumab in Metastatic Triple-Negative Breast Cancer-No Contradiction in the Eyes of a Dispassionate Observer. JAMA Oncol. 2021 Jun 24. PMID: 34165499.
- Strzebonska K, Wasylewski MT, Zaborowska L, Polak M, Slugocka E, Stras J, Blukacz M, Gyawali B, Waligora M. Risk and Benefit for Targeted Therapy Agents in Pediatric Phase II Trials in Oncology: A Systematic Review with a Meta-Analysis. Target Oncol. 2021 Jun 10. PMID: 34110559.
- Yekedüz E, Trapani D, Xu W, de Vries EG, Labaki C, Gyawali B, Gulati S, Nabhan C, Utkan G, Curigliano G, Choueiri TK, Ürün Y. Assessing Population Diversity in Phase III Trials of Cancer Drugs Supporting FDA Approval in Solid Tumors. Int J Cancer. 2021 Jun 14. PMID: 34124786.
- Gyawali B*, de Vries EGE, Dafni U, Amaral T, Barriuso J, Bogaerts J, Calles A, Curigliano G, Gomez-Roca C, Kiesewetter B, Oosting S, Passaro A, Pentheroudakis G, Piccart M, Roitberg F, Tabernero J, Tarazona N, Trapani D, Wester R, Zarkavelis G, Zielinski C, Zygoura P, Cherny NI. Biases in study design, implementation, and data analysis that distort the appraisal of clinical benefit and ESMO-Magnitude of Clinical Benefit Scale (ESMO-MCBS) scoring. ESMO Open. 2021 Apr 19;6(3):100117. PMID: 33887690. Altmetric score: 128
- Sharma S#, Wells JC, Hopman WM, Del Paggio JC, Gyawali B, Hammad N, Hay AE, Booth CM. Cancer, Clinical Trials, and Canada: Our Contribution to Worldwide Randomized Controlled Trials. Current Oncology. 2021; 28(2):1518-1527. PMID: 33924380
- Del Paggio JC, Berry JS, Hopman WM, Eisenhauer EA, Prasad V, Gyawali B and Booth C. Evolution of the Randomized Clinical Trial in the Era of Precision Oncology. JAMA Oncol. 2021. PMID: 33764385. Altmetric score: 174
- Gyawali B*, D’Andrea E, Franklin JM and Kesselheim AS. A correlation analysis to assess event-free survival as a trial-level surrogate for overall survival in early breast cancer. EClinicalMedicine Published: January 28, 2021 PMID: 33681740
- Wells JC, Sharma S, Del Paggio JC, Hopman W, Gyawali B, Mukherji D, Hammad N, Pramesh CS, Aggarwal A, Sullivan R and Booth C. An Analysis of Contemporary Oncology Randomized Clinical Trials From Low/Middle-Income vs High-Income Countries. JAMA Oncol. 2021. PMID: 33507236 Altmetric Score: 132
- Gyawali B* and West J. Lessons From ADAURA on Adjuvant Cancer Drug Trials: Evidence, Ethics, and Economics. Journal of Clinical Oncology. December 04, 2020. PMID: 33275490. Altmetric score: 90
- Gyawali B and Niraula S. Lessons from Adaptive Randomization: Spying the I-SPY2 trial in breast cancer. Journal of NCCN. November,2020. PMID: 33152697
- Desai A# and Gyawali B. Endpoints used in phase III randomized controlled trials of treatment options for COVID-19 . Eclinicalmedicine, Published by the Lancet. June 2, 2020. PMID: 32632415
- Desai A#, Kulkarni A, Rajkumar SV and Gyawali B. Clinical Trial Endpoints in Severe COVID-19. Mayo Clinic Proceedings. PMID: 32753131
- Gyawali B*, Bouche G, Crisp N. and Andre N. Challenges and opportunities for cancer clinical trials in low- and middle-income countries. Nature Cancer 1, 142–145 (2020). Altmetric score: 135
- Gyawali B*, D’Andrea E, Franklin JM and Kesselheim AS. Response Rates and Durations of Response for Biomarker-Based Cancer Drugs in Nonrandomized Versus Randomized Trials. Journal of NCCN. 2020 January. PMID: 31910385. Altmetric score: 59
- Naci H, Davis C, Savović J, Higgins JPT, Sterne JAC, Gyawali B, Romo-Sandoval X, Handley N, Booth CM. Design characteristics, risk of bias, and reporting of randomised controlled trials supporting approvals of cancer drugs by European Medicines Agency, 2014-16: cross sectional analysis. BMJ. 2019 Sep. PMID: 31533922. Altmetric Score: 396
- Linked Editorial: Flawed evidence underpins approval of new cancer drugs
- Gyawali B*, Tessema FA, Jung EH, Kesselheim AS. Assessing the Justification, Funding, Success, and Survival Outcomes of Randomized Noninferiority Trials of Cancer Drugs: A Systematic Review and Pooled Analysis. JAMA Netw Open. Published online August 30, 20192(8):e199570. PMID: 31469391 Altmetric score: 104
- Addeo A, Weiss GW and Gyawali B. Association of Industry and Academic Sponsorship With Negative Phase 3 Oncology Trials and Reported Outcomes on Participant Survival: A Pooled Analysis. JAMA Network Open 2019. PMID: 31074821 Altmetric Score: 85
- Passaro A, Spitaleri G, Gyawali B and de Marinis F. Immunotherapy in Non–Small-Cell Lung Cancer Patients With Performance Status 2: Clinical Decision Making With Scant Evidence. Journal of Clinical Oncology. 2019. PMID: 30995172
- Gyawali B* and Kesselheim AS. US Food and Drug Administration Approval of New Drugs Based on Noninferiority Trials in Oncology: A Dangerous Precedent? JAMA Oncology. 2019. PMID: 30920591 Altmetric score: 127
- Gyawali B and Prasad V. Making adjuvant therapy decisions with uncertain data. Annals of Oncology. 2019. PMID: 30715160. Altmetric score: 80
- Gyawali B*, Shimokata T, Honda K and Ando Y. Reporting harms more transparently in trials of cancer drugs. The BMJ. 2018 Nov 1;363:k4383. PMID: 30385466. Altmetric score: 365
- Hwang TJ and Gyawali B*. Association between progression-free survival and patients’ quality of life in cancer clinical trials. International Journal of Cancer 2018. PMID: 30374970. Altmetric score: 78
- Weir I.R , Marshall G.D., Schneider JI, Sherer JA, Lord EM, Gyawali B, Pasasche-Orlow MK, Benjamin EJ and Trinquart L. Interpretation of Time-to-event Outcomes in Randomized Trials: an online randomized experiment. Annals of Oncology Published online 18 October 2018. PMID: 30335127.
- With editorial by Drs. Saad ED and Tannock IF.
- Gyawali B* and Addeo A. Negative Phase 3 Randomized Controlled Trials: Why cancer drugs fail the last barrier? International Journal of Cancer. 2018 May. PMID: 29744864. Altmetric score: 63
- Gyawali B*, Hey SP and Kesselheim AS. Correlation and differences in effect sizes between progression-free survival and overall survival with PD-1 inhibitors. JAMA Network Open. June 22,2018. Altmetric score: 57
- Poster presentation at ESMO 2017. Travel grants awarded.
- Hwang TJ, Franklin JM, Chen CT, Lauffenburger JC, Gyawali B, Kesselheim AS and Darrow JJ. Efficacy, Safety, and Regulatory Approval of Food and Drug Administration–Designated Breakthrough and Nonbreakthrough Cancer Medicines. Journal of Clinical Oncology. 2018 Apr 24. PMID: 29688832
- With editorial by Nicole Kuderer and Gary Lyman.
- Gyawali B and Prasad V. Health policy: Me-too drugs with limited benefits – the tale of regorafenib for HCC. Nature Reviews Clinical Oncology. (PMID: 28719584) . Altmetric score:83.
- Gyawali B and Prasad V. Pemetrexed in Nonsquamous Non–Small-Cell Lung Cancer: The Billion Dollar Subgroup Analysis JAMA Oncology. (PMID: 28750129) . Altmetric score:105.
- Gyawali B* and Ando Y. Adjuvant Sunitinib for high-risk resected renal cell carcinoma: a meta-analysis of ASSURE and S-TRAC trials. Annals of Oncology. (2017) 28 (4): 898-899. (PMID: 27993814).
- With editorial comment by Dr. Axel Bex.
- Gyawali B.* The Imprecise Pursuit of Precision Medicine: Are biomarkers to blame? Journal of the NCCN. 2017 Jul;15(7):859-862. doi: 10.6004/jnccn/2017.0126 (PMID: 28687572). Altmetric score: 84
- Gyawali B*. The OlympiAD trial: Who Won the Gold? ecancer 2017, 11:ed75. Published December 6, 2017. PMID : 29290761
- Gyawali B and Prasad V. Combining drugs and extending treatment-a PFS endpoint is not sufficient. Nature Reviews Clinical Oncology. (PMID: 28534528) . Altmetric score:75.
- Gyawali B*, Shimokata T, Ando M, Honda K and Ando Y. Risk of Serious Adverse Events and Fatal Adverse Events with Sorafenib use in Patients with Solid Cancer: A meta-analysis of phase 3 Randomized Controlled Trials. Annals of Oncology. (2017) 28 (2): 246-253. (PMID: 27771613).
- Gyawali B and Prasad V. Drugs that lack single-agent activity: are they worth pursuing in combination? Nature Reviews Clinical Oncology. 2017 Apr;14(4):193-194. (PMID: 28266519). Altmetric score: 93
- Gyawali B* and Prasad V. Negative trials in ovarian cancer: is there such a thing as too-much optimism?Ecancermedicalscience. (PMID: 27594913).
- Author’s interview here.
- Most read commentary in the journal.
- Included in the Editor’s choice for August.
- Gyawali B and Prasad V. Same data; different interpretations. Journal of Clinical Oncology. 34, 31: 3729-3732., 2016, (PMID: 27573659). Altmetric score: 112
- 19th most read article in ASCO Journals (JCO, JOP, JGO) (as of Dec.21, 2016)
