Dr. Gyawali recently published a thought-provoking article in JNCI making a compelling case for using superiority design rather than noninferiority design to test de-escalation strategies in cancer care.
Graphical abstract by Laure-Anne Teuwen
Overtreatment is a growing concern in cancer care, where patients who may not benefit are being treated, or treatment may begin earlier, or at higher or more frequent doses, and for longer periods of time than necessary. Dr. Gyawali has previously touched on these concerns in a Medscape commentary, where he discussed overtreatment related to immunotherapy.
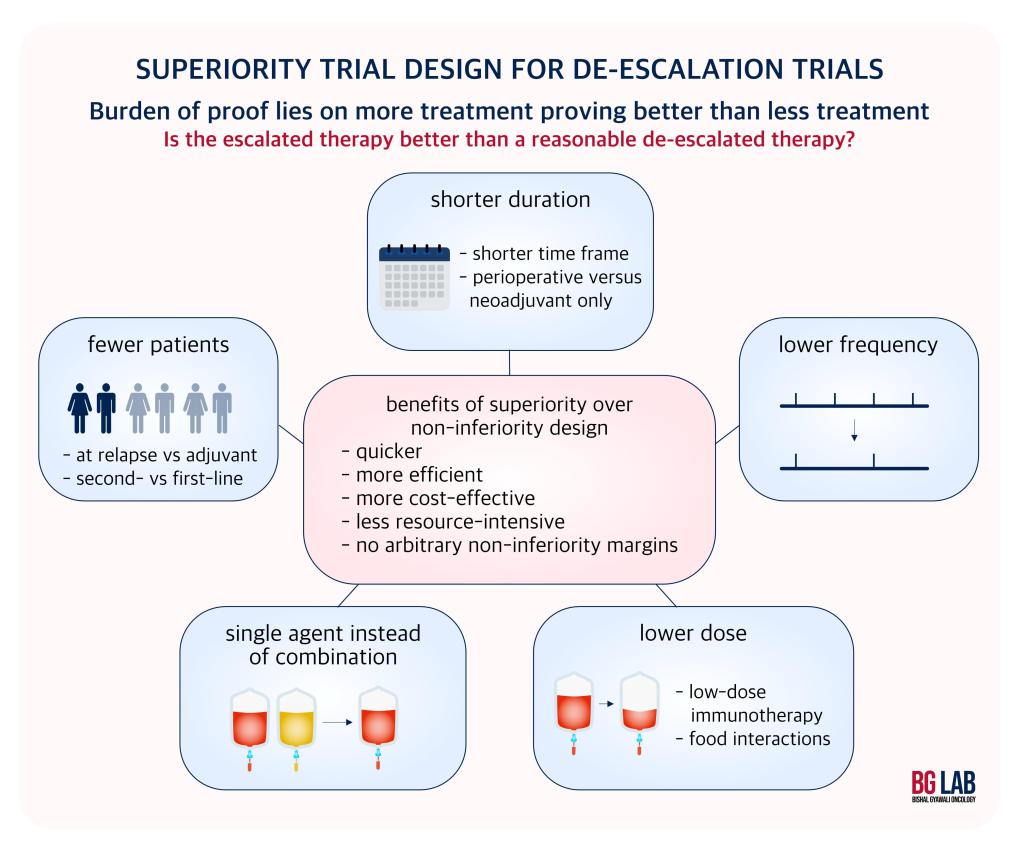
Thus, there is a growing awareness about the need to test de-escalation strategies. A de-escalated treatment would offer better quality of life, but should not compromise outcomes. Thus, classically they are tested in non-inferiority design asking whether a lower dose or lower frequency or lower duration is not worse than standard dose/frequency/duration. In the new JNCI paper, Dr. Gyawali makes a case that the burden of proof in medicine is rather to prove that the escalated treatment is superior to the de-escalated treatment, and argues that a superiority design trial should instead be employed. A superiority trial has the advantage of requiring fewer patients, and being more efficient and cost-effective.
Dr. Gyawali also argues that these trials more than recoup the cost of the trial itself due to savings in the experimental arm (some patients may never need the drug during the trial), as evidenced by the SONIA trial.
Read the full article to further grasp the nuances and evidence Dr. Gyawali provides for this argument! Please email us if you need access to the full text.
Make sure you are subscribed to BG Lab website to hear about important articles like this and more!

Leave a comment